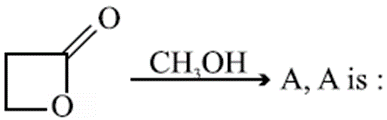
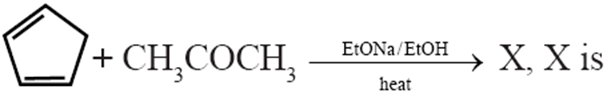Q. Which among the following statements is false ?
Solution:
(i) Osmotic pressure $\text{\pi } = \text{i} \, \, \text{C} \text{R} \text{T}$
$\therefore $ concentration is same so $\text{\pi } \propto \text{i}$ (number of ions)
$\text{i}$ for $BaCl_{2}=3, \, KCl=2,$ $ \, CH_{3}COOH=1\leq i≤2$ and for sucrose $= 1$
So, order of osmotic pressure $\text{B} \text{a} \text{C} \text{l}_{2} > \text{K} \text{C} \text{l} > \text{C} \text{H}_{3} \text{C} \text{O} \text{O} \text{H} > \text{Sucrose}$
Acetic acid does not dissociate completely. Hence, its van't Hoff factor will be in between 1 and 2.
(ii) The extent of depression in freezing point varies with the number of solute particles for a fixed solvent only, and it's a characteristic feature of the nature of solvent also $\Delta T_{f}=k_{f}\times m$
For different solvents, value of $\text{k}_{\text{f}}$ is also different so, for two different solvents the extent of depression may vary even if same number of solute particles be dissolved in them.
Questions from NTA Abhyas 2022
Chemistry Most Viewed Questions
1. If for a certain reaction $\Delta_{r}H$ is $30\, kJ\, mol^{-1}$ at $450 \,K$, the value of $\Delta_{r}S$ (in $JK^{-1}\, mol^{-1})$ for which the same reaction will be spontaneous at the same temperature is
NEET 2020
Thermodynamics
4. An organic compound contains $78 \%$ (by wt.) carbon and remaining percentage of hydrogen. The right option for the empirical formula of this compound is: [Atomic wt. of $C$ is $12, H$ is $1$]
NEET 2021
Some Basic Concepts of Chemistry
5. The density of 2 M aqueous solution of NaOH is 1.28 g/cm$^3$. The molality of the solution is
[Given that molecular mass of NaOH = 40 g mol$^{-1}$]
NEET 2019
Some Basic Concepts of Chemistry
7. In water saturated air the mole fraction of water vapour is $0.02$. If the total pressure of the saturated air is $1.2\, atm$, the partial pressure of dry air is :
NEET 2019
Solutions
9. One atom of an element weighs $ 1.8\times 10^{-22}g$ . Its atomic mass is
Manipal 2009
Some Basic Concepts of Chemistry
Latest Updates
- JEE Main 2023 February 25th Shift 1 Morning
- JEE Main 2023 February 25th Shift 2 Evening
- JEE Main 2023 January 31st Shift 1 Morning
- JEE Main 2023 January 31st Shift 2 Evening
- JEE Main 2023 January 30th Shift 1 Morning
- JEE Main 2023 January 30th Shift 2 Evening
- JEE Main 2023 January 25th Shift 1 Morning
- JEE Main 2023 January 25th Shift 2 Evening
- JEE Main 2023 January 24th Shift 1 Morning
- JEE Main 2023 January 24th Shift 2 Evening
- JEE Main 2023 February 1st Shift 1 Morning
- JEE Main 2023 February 1st Shift 2 Evening
- JEE Main 2022 July 25th Shift 1 Morning
- JEE Main 2022 July 25th Shift 2 Evening
- JEE Main 2022 July 26th Shift 1 Morning
- JEE Main 2022 July 28th Shift 1 Morning
- JEE Advanced 2022 Paper 2
- JEE Advanced 2022 Paper 1
- JEE Advanced 2021 Paper 2
- JEE Advanced 2021 Paper 1
- JEE Advanced 2020 Paper 2
- JEE Advanced 2020 Paper 1
- NEET 2022 Physics Answer Key
- NEET 2022 Chemistry Answer Key
- NEET 2022 Botany Biology Answer Key
- NEET 2022 Zoology Biology Answer Key
- NEET Rank Predictor 2023