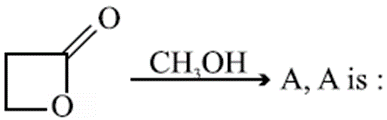
Q. The rapid change of pH near the stoichiometric point of an acid-base titration is the basis of indicator detection. pH of the solution is related to ratio of the concentration of the conjugate acid (HIn) and base $\left(\right.In^{-}\left.\right)$ forms of the indicator by the expression
Solution:
Let us consider the formation of a salt of a weak acid and a strong base.
$In^{-}+H_{2}O\rightleftharpoonsHIn+OH^{-}$
$K_{h}\frac{\left[\right. H I n \left]\right. \left[\right. O H^{-} \left]\right.}{\left[\right. I n^{-} \left]\right.}$ …(i)
Other equations present in the solution are
$HIn\rightleftharpoonsH^{+}+In^{-}$
$H_{2}O\rightleftharpoonsH^{+}+OH^{-}$
$K_{I n}=\frac{\left[\right. H^{+} \left]\right. \left[\right. I n^{-} \left]\right.}{\left[\right. H I n \left]\right.}$ …(ii)
$K_{w}=\left[\right.H^{+}\left]\right.\left[\right.OH^{-}\left]\right.$ …(iii)
From (ii) and (iii),
$\frac{K_{w}}{K_{I n}}\frac{\left[\right. H I n \left]\right. \left[\right. O H^{-} \left]\right.}{\left[\right. I n^{-} \left]\right.}=K_{h}$
$\left[\right.OH^{-}\left]\right.=\frac{K_{w}}{K_{I n}}\frac{\left[\right. I n^{-} \left]\right.}{\left[\right. H I n \left]\right.}$
$log\left[\right.OH^{-}\left]\right.=logK_{w}-logK_{I n}+log\frac{\left[\right. I n^{-} \left]\right.}{\left[\right. H I n \left]\right.}$
$-pOH=-pK_{w}+pK_{I n}+log\frac{\left[\right. I n^{-} \left]\right.}{\left[\right. H I n \left]\right.}$
$pK_{w}-pOH=pK_{I n}+log\frac{\left[\right. I n^{-} \left]\right.}{\left[\right. H I n \left]\right.}$
or, $pH=pK_{I n}+log\frac{\left[\right. I n^{-} \left]\right.}{\left[\right. H I n \left]\right.}$
i.e. $log\frac{\left[\right. I n^{-} \left]\right.}{\left[\right. H I n \left]\right.}=pH-pK_{I n}$
Questions from NTA Abhyas 2022
Chemistry Most Viewed Questions
1. If for a certain reaction $\Delta_{r}H$ is $30\, kJ\, mol^{-1}$ at $450 \,K$, the value of $\Delta_{r}S$ (in $JK^{-1}\, mol^{-1})$ for which the same reaction will be spontaneous at the same temperature is
NEET 2020
Thermodynamics
4. An organic compound contains $78 \%$ (by wt.) carbon and remaining percentage of hydrogen. The right option for the empirical formula of this compound is: [Atomic wt. of $C$ is $12, H$ is $1$]
NEET 2021
Some Basic Concepts of Chemistry
5. The density of 2 M aqueous solution of NaOH is 1.28 g/cm$^3$. The molality of the solution is
[Given that molecular mass of NaOH = 40 g mol$^{-1}$]
NEET 2019
Some Basic Concepts of Chemistry
7. In water saturated air the mole fraction of water vapour is $0.02$. If the total pressure of the saturated air is $1.2\, atm$, the partial pressure of dry air is :
NEET 2019
Solutions
9. One atom of an element weighs $ 1.8\times 10^{-22}g$ . Its atomic mass is
Manipal 2009
Some Basic Concepts of Chemistry
Latest Updates
- JEE Main 2023 February 25th Shift 1 Morning
- JEE Main 2023 February 25th Shift 2 Evening
- JEE Main 2023 January 31st Shift 1 Morning
- JEE Main 2023 January 31st Shift 2 Evening
- JEE Main 2023 January 30th Shift 1 Morning
- JEE Main 2023 January 30th Shift 2 Evening
- JEE Main 2023 January 25th Shift 1 Morning
- JEE Main 2023 January 25th Shift 2 Evening
- JEE Main 2023 January 24th Shift 1 Morning
- JEE Main 2023 January 24th Shift 2 Evening
- JEE Main 2023 February 1st Shift 1 Morning
- JEE Main 2023 February 1st Shift 2 Evening
- JEE Main 2022 July 25th Shift 1 Morning
- JEE Main 2022 July 25th Shift 2 Evening
- JEE Main 2022 July 26th Shift 1 Morning
- JEE Main 2022 July 28th Shift 1 Morning
- JEE Advanced 2022 Paper 2
- JEE Advanced 2022 Paper 1
- JEE Advanced 2021 Paper 2
- JEE Advanced 2021 Paper 1
- JEE Advanced 2020 Paper 2
- JEE Advanced 2020 Paper 1
- NEET 2022 Physics Answer Key
- NEET 2022 Chemistry Answer Key
- NEET 2022 Botany Biology Answer Key
- NEET 2022 Zoology Biology Answer Key
- NEET Rank Predictor 2023