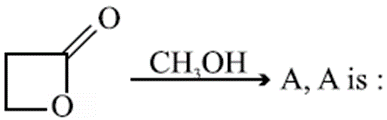
Q. The electrolytes usually used in the electroplating of gold and silver, respectively, are
Solution:
Amine complexes of gold are not stable, so, they cannot be used as a solvent.Moreover, in electroplating with silver, silver cyanide is used as electrolyte because the cyanide ion, $CN^{-}$ , reacts with silver ion, $Ag^{+}$ to form the complex ion $Ag\left(\right.CN\left.\right)_{2}^{-}$ . This produces a silver and more adherent silver plating.
Questions from NTA Abhyas 2022
Chemistry Most Viewed Questions
1. If for a certain reaction $\Delta_{r}H$ is $30\, kJ\, mol^{-1}$ at $450 \,K$, the value of $\Delta_{r}S$ (in $JK^{-1}\, mol^{-1})$ for which the same reaction will be spontaneous at the same temperature is
NEET 2020
Thermodynamics
4. An organic compound contains $78 \%$ (by wt.) carbon and remaining percentage of hydrogen. The right option for the empirical formula of this compound is: [Atomic wt. of $C$ is $12, H$ is $1$]
NEET 2021
Some Basic Concepts of Chemistry
5. The density of 2 M aqueous solution of NaOH is 1.28 g/cm$^3$. The molality of the solution is
[Given that molecular mass of NaOH = 40 g mol$^{-1}$]
NEET 2019
Some Basic Concepts of Chemistry
7. In water saturated air the mole fraction of water vapour is $0.02$. If the total pressure of the saturated air is $1.2\, atm$, the partial pressure of dry air is :
NEET 2019
Solutions
9. One atom of an element weighs $ 1.8\times 10^{-22}g$ . Its atomic mass is
Manipal 2009
Some Basic Concepts of Chemistry
Latest Updates
- JEE Main 2023 February 25th Shift 1 Morning
- JEE Main 2023 February 25th Shift 2 Evening
- JEE Main 2023 January 31st Shift 1 Morning
- JEE Main 2023 January 31st Shift 2 Evening
- JEE Main 2023 January 30th Shift 1 Morning
- JEE Main 2023 January 30th Shift 2 Evening
- JEE Main 2023 January 25th Shift 1 Morning
- JEE Main 2023 January 25th Shift 2 Evening
- JEE Main 2023 January 24th Shift 1 Morning
- JEE Main 2023 January 24th Shift 2 Evening
- JEE Main 2023 February 1st Shift 1 Morning
- JEE Main 2023 February 1st Shift 2 Evening
- JEE Main 2022 July 25th Shift 1 Morning
- JEE Main 2022 July 25th Shift 2 Evening
- JEE Main 2022 July 26th Shift 1 Morning
- JEE Main 2022 July 28th Shift 1 Morning
- JEE Advanced 2022 Paper 2
- JEE Advanced 2022 Paper 1
- JEE Advanced 2021 Paper 2
- JEE Advanced 2021 Paper 1
- JEE Advanced 2020 Paper 2
- JEE Advanced 2020 Paper 1
- NEET 2022 Physics Answer Key
- NEET 2022 Chemistry Answer Key
- NEET 2022 Botany Biology Answer Key
- NEET 2022 Zoology Biology Answer Key
- NEET Rank Predictor 2023