- Tardigrade
- Question
- Chemistry
- Which of the following statements is/ are true? (I) Ethers are soluble in conc. H2SO4 but separate out on addition of water (II) Ethers are used as solvents for BF3 and Grignard reagent (III) Mononitration of p-methylanisole gives 2-nitro-4 methylanisole (IV) Monobromination of p-ethoxyphenol gives 2-bromo-4-ethoxyphenol (V) 4-Chlorophenol (I) will dissolve in NaOH but 4-chloro-1 -methyl benzene (II) will not (VI) 4-Methyl benzoic (III) acid will dissolve in aq. NaHCO3 but 4-methyl phenol (IV) will not (VII) 2,4,6-Trinitrophenol (V) will dissolve in aq. NaHCO3 but 4-methyl phenol (VI) will not (VIII) 4 -Ethyl phenol (VII) will dissolve in aq. NaOH but ethyl phenyl ether (VIII) will not
Q.
Which of the following statements is/ are true?
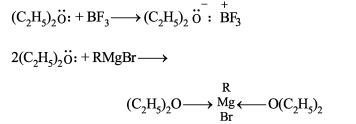
(I) Ethers are soluble in conc. but separate out on addition of water
(II) Ethers are used as solvents for and Grignard reagent
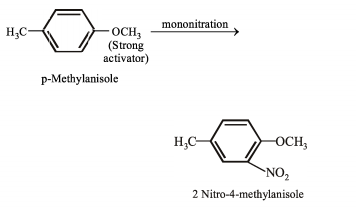
(III) Mononitration of -methylanisole gives -nitro- methylanisole
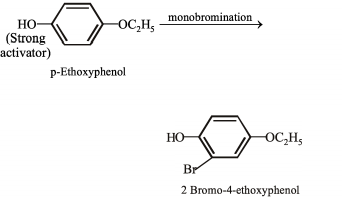
(IV) Monobromination of -ethoxyphenol gives -bromo--ethoxyphenol
(V) -Chlorophenol (I) will dissolve in but -chloro- -methyl benzene (II) will not
(VI) -Methyl benzoic (III) acid will dissolve in aq. but -methyl phenol (IV) will not
(VII) -Trinitrophenol (V) will dissolve in aq. but -methyl phenol (VI) will not
(VIII) -Ethyl phenol (VII) will dissolve in aq. but
ethyl phenyl ether (VIII) will not
Answer: 8
Solution: