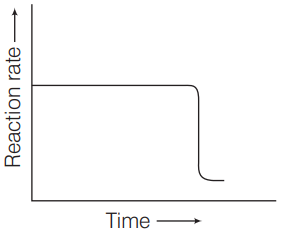
Q. Which of the following graphs are correct for a zero order reaction?
Solution:
For a zero order reaction,
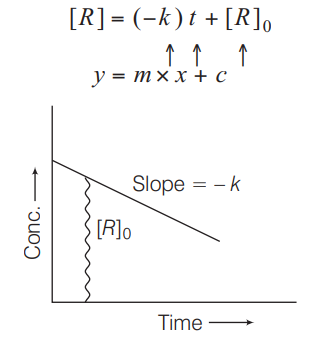
On comparing with equation of straight line concentration
time
Slope rate constant
Intercept initial concentration
On rearranging Eq. (i)
Rate