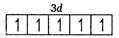
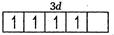
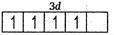
Q. What is the correct order of spin only magnetic moment (in BM) of and ?
Solution:
Spin only magnetic moment depends upon die number of unpaired electrons, more the number of unpaired electrons, greater will be the spin only magnetic moment.
Number of unpaired electrons =5
Number of unpaired electrons =4
Number of unpaired electrons =3
So, the correct order of spin only magnetic moment is