Q. What is the correct order of spin only magnetic moment (in BM) of $ M{{n}^{2+}},C{{r}^{2+}} $ and $ {{V}^{2+}} $ ?
Jharkhand CECEJharkhand CECE 2009
Solution:
Spin only magnetic moment depends upon die number of unpaired electrons, more the number of unpaired electrons, greater will be the spin only magnetic moment.
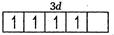
$ _{25}Mn=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{5}},4{{s}^{2}} $
$ M{{n}^{2+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{5}},4{{s}^{o}} $
Number of unpaired electrons =5
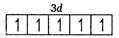
$ {{\,}_{24}}Cr=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{d}^{5}},4{{s}^{1}} $
$ C{{r}^{2+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{4}},4{{s}^{0}} $
Number of unpaired electrons =4
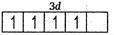
Number of unpaired electrons =3
So, the correct order of spin only magnetic moment is
$ M{{n}^{2+}} > C{{r}^{2+}} > {{V}^{2+}} $