- Tardigrade
- Question
- Chemistry
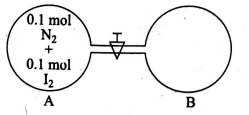
- Two vessels of equal volume are connected to each other by a value of negligible volume. One of the containers has 2.8 g of N 2 12.7 g of I2 at a temperature T1 . The other container is completely evacuated. The container that has N2 and I2 is heated to temperature T2 while the evacuated container is heated T2 / 3 . The value is now opened. Calculate the mass of N 2 in container (B) after a very long time I2 sublimes at T2. (report your answer in nearest integer form in grams)
Q. Two vessels of equal volume are connected to each other by a value of negligible volume. One of the containers has of of at a temperature The other container is completely evacuated. The container that has and is heated to temperature while the evacuated container is heated The value is now opened. Calculate the mass of in container (B) after a very long time sublimes at . (report your answer in nearest integer form in grams)
Answer: 2
Solution: