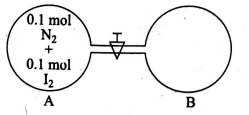
Q. Two vessels of equal volume are connected to each other by a value of negligible volume. One of the containers has $2.8\, g$ of $N _{2} 12.7\, g$ of $I_{2}$ at a temperature $T_{1} .$ The other container is completely evacuated. The container that has $N_{2}$ and $I_{2}$ is heated to temperature $T_{2}$ while the evacuated container is heated $T_{2} / 3 .$ The value is now opened. Calculate the mass of $N _{2}$ in container (B) after a very long time $I_{2}$ sublimes at $T_{2}$. (report your answer in nearest integer form in grams)
States of Matter
Solution: