- Tardigrade
- Question
- Chemistry
- The wave function, ψ n , l, m ℓ is a mathematical function whose value depends upon spherical polar coordinates (r, θ, φ ) of the electron and characterized by the quantum numbers n , ℓ and m l . Here r is distance from nucleus, θ is colatitude and φ is azimuth. In the mathematical functions given in the Table, Z is atomic number and a0 is Bohr radius. Column 1 Column 2 Column 3 (I) 1s orbital (i) ψn, l, ml propto((Z/ae))(3/2) e-((z/a0)) (p) <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/chemistry/adff06bac6591288720970fc0243d3ed-.png /> (II) 2s orbital (ii) one radial node (Q) Probability density at nucleus propto (1/a30) (III) 2pz orbital (iii) ψn, l, ml propto((Z/ao))(5/2) r e-((z/2 a0)) cos θ (R) Probability density is maximum at nucleus (IV) 3d2z Orbital (iv) xy-plane is a nodal plane (S) Energy needed to excite electron from n=2 state to n= 4 state is (27/32) times the energy needed to excite electron from n=2 state to n=6 state For the given orbital in Column 1, the only CORRECT combination for any hydrogen like species is
Q.
The wave function, is a mathematical function whose value depends upon spherical polar coordinates ( ) of the electron and characterized by the quantum numbers and Here is distance from nucleus, is colatitude and is azimuth. In the mathematical functions given in the Table, is atomic number and is Bohr radius.
Column 1
Column 2
Column 3
(I) 1s orbital
(i)
(p) 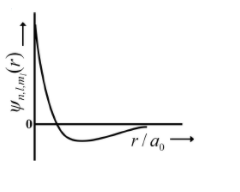
(II) 2s orbital
(ii) one radial node
(Q) Probability density at nucleus
(III) orbital
(iii)
(R) Probability density is maximum at nucleus
(IV) Orbital
(iv) xy-plane is a nodal plane
(S) Energy needed to excite electron from state to state is times the energy needed to excite electron from state to state
For the given orbital in Column 1, the only CORRECT combination for any hydrogen like species is
| Column 1 | Column 2 | Column 3 |
|---|---|---|
| (I) 1s orbital | (i) | (p)  |
| (II) 2s orbital | (ii) one radial node | (Q) Probability density at nucleus |
| (III) orbital | (iii) | (R) Probability density is maximum at nucleus |
| (IV) Orbital | (iv) xy-plane is a nodal plane | (S) Energy needed to excite electron from state to state is times the energy needed to excite electron from state to state |
Solution:
