- Tardigrade
- Question
- Chemistry
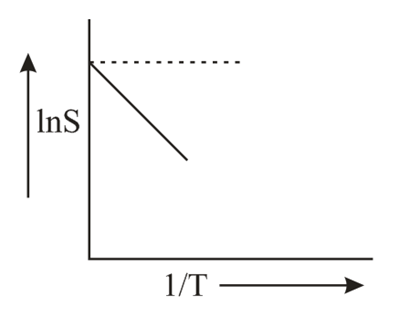
- The solubility (S) of a solute in water varies with temperature as given by S=Ae- Δ H / R T,Δ H being the enthalpy of solution. For a given solute, variation of lnS with temperature is as shown in figure. The solute is expected to be <img class=img-fluid question-image alt=Question src=https://cdn.tardigrade.in/q/nta/c-aa1tfheqlkl5eain.jpg />
Q.
The solubility (S) of a solute in water varies with temperature as given by being the enthalpy of solution. For a given solute, variation of lnS with temperature is as shown in figure. The solute is expected to be
Solution: