Q.
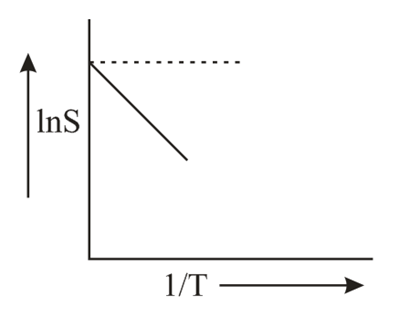
The solubility (S) of a solute in water varies with temperature as given by $S=Ae^{- \Delta H / R T},\Delta H$ being the enthalpy of solution. For a given solute, variation of lnS with temperature is as shown in figure. The solute is expected to be
NTA AbhyasNTA Abhyas 2022
Solution: