- Tardigrade
- Question
- Chemistry
- The rate of the reaction <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/chemistry/e5b25b4660ce650de6fd5711ae0eafe3-.png /> is influenced by the hyperconjugation effect of group R. If R sequentially is I. CH 3- II. CH 3- CH 2- III. H3C - undersetCH underset|CH3 Iv.<img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/chemistry/d199263612e14bbc215dee0604d01543-.png /> the increasing order of speed of the above reaction is
Q.
The rate of the reaction
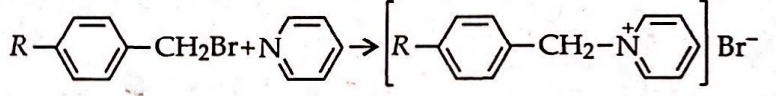
is influenced by the hyperconjugation effect of group . If sequentially is
I.
II.
III.
Iv.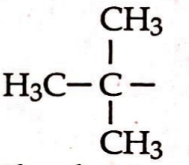
the increasing order of speed of the above reaction is
Solution: