Q.
The rate of the reaction
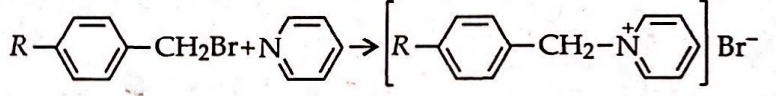
is influenced by the hyperconjugation effect of group $R$. If $R$ sequentially is
I. $\quad CH _{3}-$
II. $CH _{3}- CH _{2}-$
III. $H_3C -\underset{CH}{\underset{|}{CH_3}}$
Iv.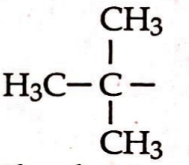
the increasing order of speed of the above reaction is
Organic Chemistry – Some Basic Principles and Techniques
Solution: