- Tardigrade
- Question
- Chemistry
- The polymerization of propene to linear polypropene is represented by the reaction <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/chemistry/024ce993cc1e97189001f4836eb6622c-.png /> Where n has large integral value, the average enthalpies of bond dissociation for (C=C) and (C-C) at 298 K are +590 and +331 kJ mol -1, respectively. The enthalpy of polymerization is -360 kJ -1. Find the value of n
Q.
The polymerization of propene to linear polypropene is represented by the reaction
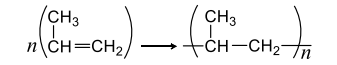
Where has large integral value, the average enthalpies of bond dissociation for and at are and , respectively. The enthalpy of polymerization is . Find the value of
Answer: 5
Solution: