Q.
The polymerization of propene to linear polypropene is represented by the reaction
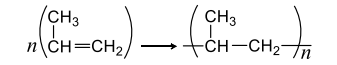
Where $n$ has large integral value, the average enthalpies of bond dissociation for $(C=C)$ and $(C-C)$ at $298 \,K$ are $+590$ and $+331 \,kJ \,mol ^{-1}$, respectively. The enthalpy of polymerization is $-360 \,kJ ^{-1}$. Find the value of $n$
Thermodynamics
Solution: