- Tardigrade
- Question
- Physics
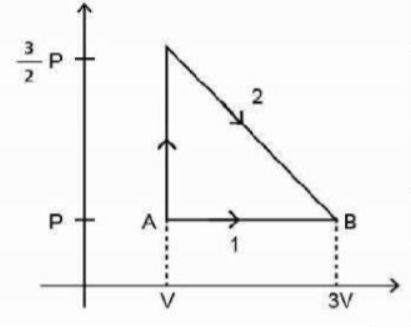
- The P-V diagram shown below indicates two paths along which a sample of gas can be taken from state A to state B. The energy equal to 5 PV in the form of heat is required to be transferred if the Path -1 is chosen. How much energy in the form of heat should be transferred if Path-2 is chosen?
Q.
The diagram shown below indicates two paths along which a sample of gas can be taken from state to state . The energy equal to in the form of heat is required to be transferred if the Path is chosen. How much energy in the form of heat should be transferred if Path- is chosen?
Solution:
