- Tardigrade
- Question
- Chemistry
- The figure below is the plot of potential energy versus internuclear distance (d) of H 2 molecule in the electronic ground state. What is the value of the net potential energy E0 (as indicated in figure) in kJ mol -1, for d=d0 at which the electron-electron repulsion and the nucleus-nucleus repulsion energies are absent? As reference, the potential energy of H atom is taken as zero when its electron and the nucleus are infinitely far apart. Use Avogadro constant as 6.023 × 1023 mol -1.
Q.
The figure below is the plot of potential energy versus internuclear distance of molecule in the electronic ground state. What is the value of the net potential energy (as indicated in figure) in , for at which the electron-electron repulsion and the nucleus-nucleus repulsion energies are absent? As reference, the potential energy of atom is taken as zero when its electron and the nucleus are infinitely far apart.
Use Avogadro constant as .
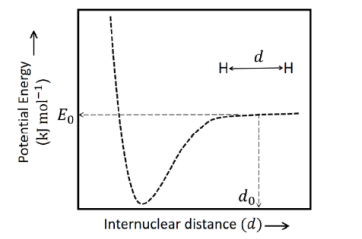
Answer: -5246.50
Solution: