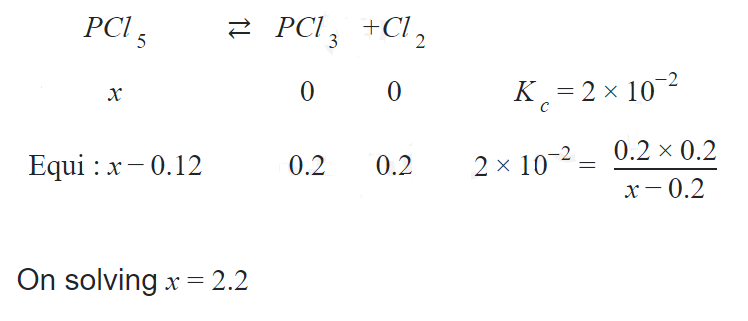- Tardigrade
- Question
- Chemistry
- The equilibrium constant for the equilibrium PCl5(g) <=> PCl3(g) + Cl2(g) at a particular temperature is 2 × 10-2mol dm-3. The number of moles of PCl5 that must be taken in a one-litre flask at the same temperature to obtain a concentration of 0.20 mol of chlorine at equilibrium is
Q. The equilibrium constant for the equilibrium at a particular temperature is mol . The number of moles of that must be taken in a one-litre flask at the same temperature to obtain a concentration of mol of chlorine at equilibrium is
Solution: