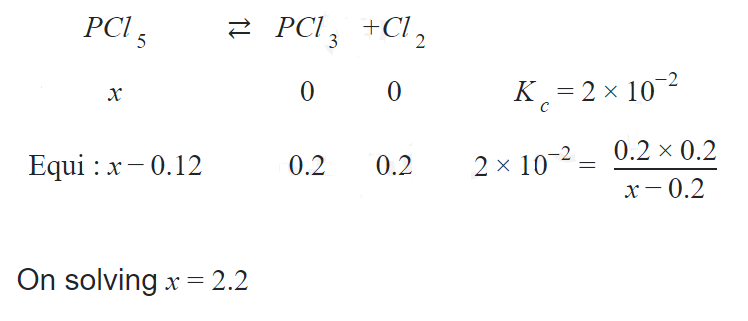Q. The equilibrium constant for the equilibrium $PCl_5(g) \ce{<=>} PCl_3(g) + Cl_2(g)$ at a particular temperature is $2 \times 10^{-2}$mol $dm^{-3}$. The number of moles of $PCl_5$ that must be taken in a one-litre flask at the same temperature to obtain a concentration of $0.20$ mol of chlorine at equilibrium is
KEAMKEAM 2020
Solution: