- Tardigrade
- Question
- Chemistry
- Study the given diagram of the periodic table and fill up the blanks with appropriate choice. <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/chemistry/33ee28e0317c2dd12fa2c18cb3fd8120-.jpeg /> (→ indicates the increasing trend of property.) P Q R S (a) Ionisation enthalpy Electron gain enthalpy Atomic radius Electro-negativity (b) Atomic radius Ionisation enthalpy Electro-negativity Electron gain enthalpy (c) Ionisation enthalpy Atomic radius Electro-negativity Electron gain enthalpy (d) Electro-negativity Electron gain enthalpy Ionisation enthalpy Atomic radius
Q.
Study the given diagram of the periodic table and fill up the blanks with appropriate choice.
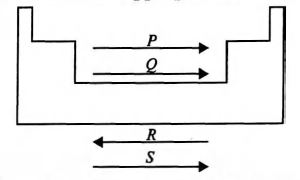
( indicates the increasing trend of property.)
P
Q
R
S
(a)
Ionisation enthalpy
Electron gain
enthalpy
Atomic radius
Electro-negativity
(b)
Atomic radius
Ionisation enthalpy
Electro-negativity
Electron gain
enthalpy
(c)
Ionisation enthalpy
Atomic radius
Electro-negativity
Electron gain
enthalpy
(d)
Electro-negativity
Electron gain
enthalpy
Ionisation enthalpy
Atomic radius
| P | Q | R | S | |
|---|---|---|---|---|
| (a) | Ionisation enthalpy | Electron gain enthalpy | Atomic radius | Electro-negativity |
| (b) | Atomic radius | Ionisation enthalpy | Electro-negativity | Electron gain enthalpy |
| (c) | Ionisation enthalpy | Atomic radius | Electro-negativity | Electron gain enthalpy |
| (d) | Electro-negativity | Electron gain enthalpy | Ionisation enthalpy | Atomic radius |
Solution: