Q.
Study the given diagram of the periodic table and fill up the blanks with appropriate choice.
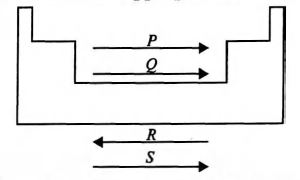
($\to$ indicates the increasing trend of property.)
P
Q
R
S
(a)
Ionisation enthalpy
Electron gain
enthalpy
Atomic radius
Electro-negativity
(b)
Atomic radius
Ionisation enthalpy
Electro-negativity
Electron gain
enthalpy
(c)
Ionisation enthalpy
Atomic radius
Electro-negativity
Electron gain
enthalpy
(d)
Electro-negativity
Electron gain
enthalpy
Ionisation enthalpy
Atomic radius
| P | Q | R | S | |
|---|---|---|---|---|
| (a) | Ionisation enthalpy | Electron gain enthalpy | Atomic radius | Electro-negativity |
| (b) | Atomic radius | Ionisation enthalpy | Electro-negativity | Electron gain enthalpy |
| (c) | Ionisation enthalpy | Atomic radius | Electro-negativity | Electron gain enthalpy |
| (d) | Electro-negativity | Electron gain enthalpy | Ionisation enthalpy | Atomic radius |
Classification of Elements and Periodicity in Properties
Solution: