Q.
One mole of a mono-atomic gas undergoes the processes and as shown. The corresponding graph for the above process for moles of the gas is
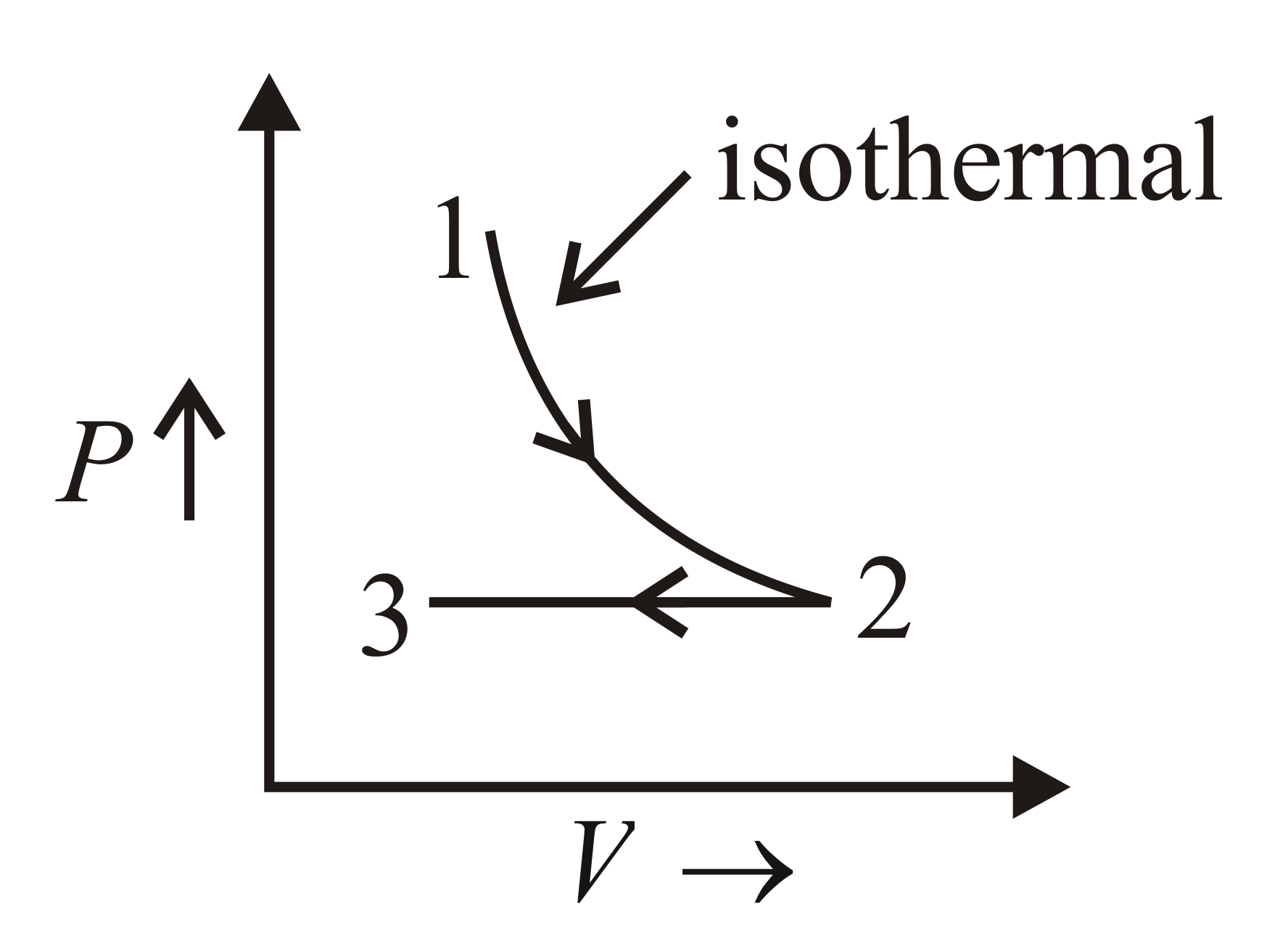
Solution:
Process is an isothermal process. So, temperature is constant.
Process is an isobaric process. So, pressure and is constant.
Change in number of moles of the gas does not affect the nature of graph.
Process is an isobaric process. So, pressure and is constant.
Change in number of moles of the gas does not affect the nature of graph.