Q.
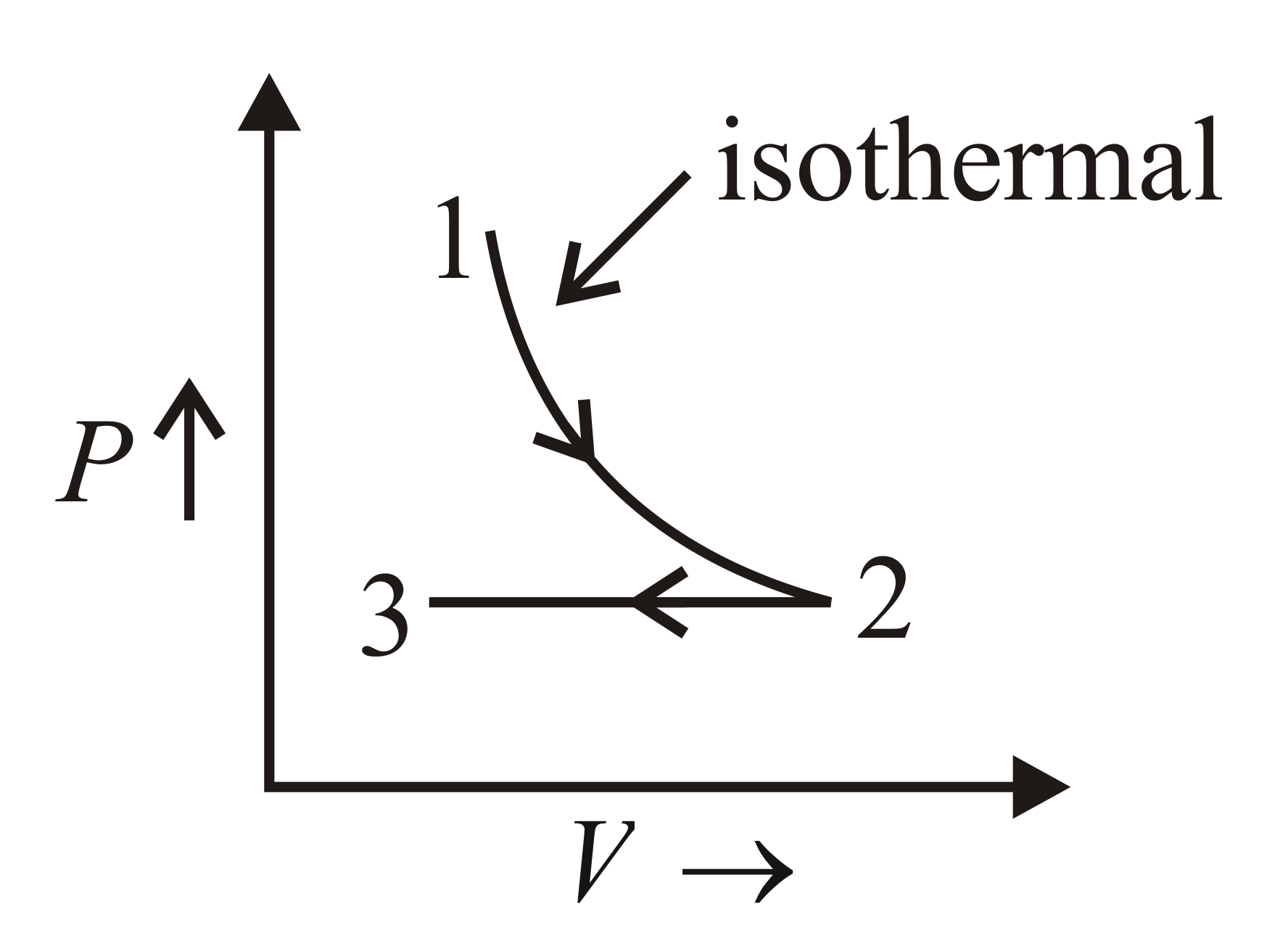
One mole of a mono-atomic gas undergoes the processes $1-2$ and $2-3$ as shown. The corresponding graph for the above process for $2$ moles of the gas is
NTA AbhyasNTA Abhyas 2022
Solution:
Process $1-2$ is an isothermal process. So, temperature $T$ is constant.
Process $2-3$ is an isobaric process. So, pressure $P$ and $\frac{T}{V}$ is constant.
Change in number of moles of the gas does not affect the nature of graph.
Process $2-3$ is an isobaric process. So, pressure $P$ and $\frac{T}{V}$ is constant.
Change in number of moles of the gas does not affect the nature of graph.