- Tardigrade
- Question
- Physics
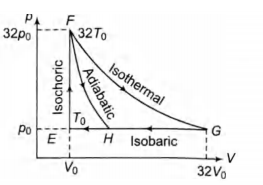
- One mole of a monatomic ideal gas is taken along two cyclic processes E arrow F arrow G arrow E and E arrow F arrow H arrow E as shown in the p-V diagram. The processes involved are purely isochoric, isobaric, isothermal or adiabatic. Match the paths in List I with the magnitudes of the work done in List II and select the correct answer using the codes given below the lists. <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/physics/25b5fc9c4a5ef50deb7df498c8c3dcc1-.png /> List I List II P. G → E 1. 160 p0V0 In 2 Q. G → H 2 36 P0V0 R. F → H 3. 24 P0V0 S. F → G 4. 31 P0V0
Q.
One mole of a monatomic ideal gas is taken along two cyclic processes E F G E and E F H E as shown in the p-V diagram.
The processes involved are purely isochoric, isobaric, isothermal or adiabatic.
Match the paths in List I with the magnitudes of the work done in List II and select the correct answer using the codes given below the lists.

List I
List II
P.
1.
In 2
Q.
2
R.
3.
S.
4.
| List I | List II | ||
|---|---|---|---|
| P. | 1. | In 2 | |
| Q. | 2 | ||
| R. | 3. | ||
| S. | 4. | ||
Solution:
In F G work done in isothermal process is
in G
in G work done is less than
in F work done is 36 p