- Tardigrade
- Question
- Chemistry
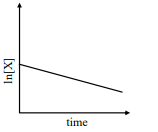
- Match the rate expressions in LIST-I for the decomposition of X with the corresponding profiles provided in LIST-II. X s and k constants having appropriate units. LIST I LIST II I text rate =( k [ X ]/ X s +[ X ]) under all possible initial concentration of X i <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/chemistry/09924ad1f61b739b7be9f59ca630b43a-.png /> II text rate =( k [ X ]/ X s +[ X ]) where initial concentration of X are much less than X s ii <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/chemistry/8b966d5e6dbd38473a0c07bfcd1ac028-.png /> III text rate =( k [ X ]/ X s +[ X ]) where initial concentration of X are much higher than X s iii <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/chemistry/454e0bd0ccd01c3e27da515449c685a3-.png /> IV text rate =( k [ X ]2/ X s +[ X ]) ,br/>where initial concentration of X is much higher than X s iv <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/chemistry/a22a8ebef7c515deae3d3306c8b0488a-.png /> v <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/chemistry/80d24ffcc5a90f88422ed580318c67ac-.png />
Q.
Match the rate expressions in LIST-I for the decomposition of with the corresponding profiles provided in LIST-II. and constants having appropriate units.
LIST I
LIST II
I
under all possible initial concentration of
i
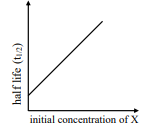
II
where initial concentration of are much less than
ii
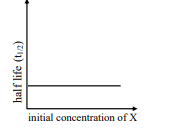
III
where initial concentration of are much higher than
iii
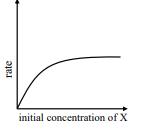
IV
,br/>where initial concentration of is much higher than
iv
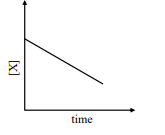
v
| LIST I | LIST II | ||
|---|---|---|---|
| I | under all possible initial concentration of |
i |  |
| II | where initial concentration of are much less than |
ii |  |
| III | where initial concentration of are much higher than |
iii |  |
| IV | ,br/>where initial concentration of is much higher than | iv |  |
| v |  |
||
Solution: