- Tardigrade
- Question
- Chemistry
- Match the graphs given in column I with the order given in column II and mark the appropriate choice. Column I Column II (A) <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/physics/569a05e74695edc46d4327f450e781f4-.png /> (i) Third order (B) <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/physics/ad4ad211c73433732f2fe2bdadaf8d61-.png /> (ii) First order (C) <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/physics/ff3584ac000e34faa55b74b501355123-.png /> (iii) Zero order (D) <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/physics/1e98ecea9ce07b83485ffa845491e185-.png /> (iv) Second order
Q.
Match the graphs given in column I with the order given in column II and mark the appropriate choice.
Column I
Column II
(A)

(i)
Third order
(B)
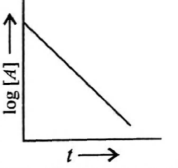
(ii)
First order
(C)

(iii)
Zero order
(D)

(iv)
Second order
| Column I | Column II | ||
|---|---|---|---|
| (A) |  |
(i) | Third order |
| (B) |  |
(ii) | First order |
| (C) |  |
(iii) | Zero order |
| (D) |  |
(iv) | Second order |
Solution: