Q.
Match the graphs given in column I with the order given in column II and mark the appropriate choice.
Column I
Column II
(A)

(i)
Third order
(B)
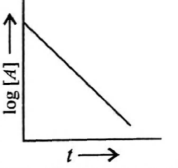
(ii)
First order
(C)

(iii)
Zero order
(D)

(iv)
Second order
| Column I | Column II | ||
|---|---|---|---|
| (A) |  |
(i) | Third order |
| (B) |  |
(ii) | First order |
| (C) |  |
(iii) | Zero order |
| (D) |  |
(iv) | Second order |
Chemical Kinetics
Solution: