- Tardigrade
- Question
- Physics
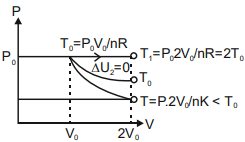
- <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/physics/57493acb338324b24349bdb871e8d3ad-.png /> In the given figure, 1 represents isobaric, 2 represents isothermal and 3 represents adiabatic processes of an ideal gas. If Δ U1, Δ U2, Δ U3 be the changes in internal energy in these processes respectively, then
Q.
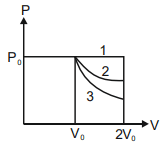
In the given figure, represents isobaric, represents isothermal and represents adiabatic processes of an ideal gas. If be the changes in internal energy in these processes respectively, then
Solution: