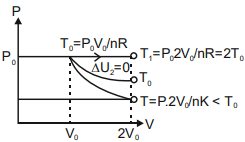
Q.
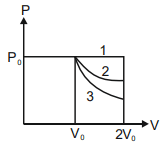
In the given figure, $1$ represents isobaric, $2$ represents isothermal and $3$ represents adiabatic processes of an ideal gas. If $\Delta U_{1}, \Delta U_{2}, \Delta U_{3}$ be the changes in internal energy in these processes respectively, then
WBJEEWBJEE 2021
Solution: