- Tardigrade
- Question
- Chemistry
- If enthalpy of hydrogenation of ( C )6( H )6( l ) into ( C )6( H )12( l ) is -205 k J and resonance energy of ( C )6( H )6( l ) is -152 kJ mol -1 then enthalpy of hydrogenation of <img class=img-fluid question-image alt=Question src=https://cdn.tardigrade.in/q/nta/c-zob7jkbvdcta4dmq.jpg /> (l) to ( C )6( H )12( l ) is? [Assume enthalpies of vapourisation of all liquids involved are equal].
Q.
If enthalpy of hydrogenation of into is and resonance energy of is then enthalpy of hydrogenation of
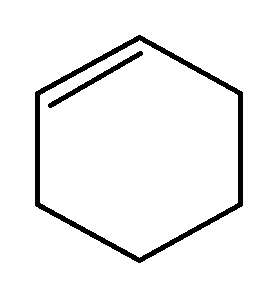
(l) to is? [Assume enthalpies of vapourisation of all liquids involved are equal].
Solution: