Q.
If enthalpy of hydrogenation of $( C )_{6}( H )_{6}( l )$ into $( C )_{6}( H )_{12}( l )$ is $-205 \,k \,J$ and resonance energy of $( C )_{6}( H )_{6}( l )$ is $-152\, kJ\, mol ^{-1}$ then enthalpy of hydrogenation of
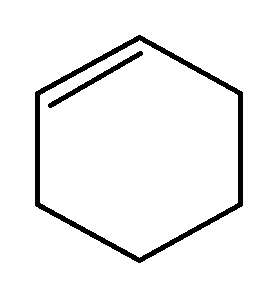
(l) to $( C )_{6}( H )_{12}( l )$ is? [Assume enthalpies of vapourisation of all liquids involved are equal].
NTA AbhyasNTA Abhyas 2022
Solution: