- Tardigrade
- Question
- Chemistry
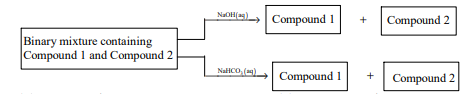
- Identify the binary mixture(s) that can be separated into individual compounds, by differential extraction, as shown in the given scheme. <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/chemistry/c55dbd0fcb368e9788186163994809ac-.png />
Q.
Identify the binary mixture(s) that can be separated into individual compounds, by differential extraction, as
shown in the given scheme.
Solution: