Q.
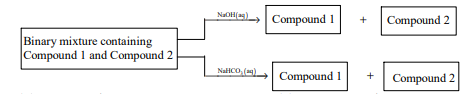
Identify the binary mixture(s) that can be separated into individual compounds, by differential extraction, as
shown in the given scheme.
JEE AdvancedJEE Advanced 2012
Solution:

Solution: