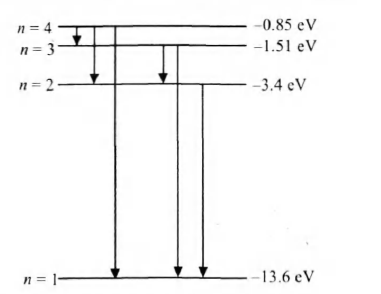
Q. Hydrogen atom in ground state is excited by a monochromatic radiation of . Number of spectral lines in the resulting spectrum emitted will be
Solution:
Energy of the photon,
After absorbing a photon of energy , the electron will reach to third excited state of energy ,
since energy difference corresponding
to and is .
Number of spectral lines emitted