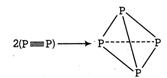
Q. Given the following bond dissociation enthalpies . Compare the enthalpy changes for the process (i) (ii) Choose the correct option.
Solution:
The process involves replacement of units by 6 X?X bonds in the tetrahedron. Energy is released when bonds are formed and thus species formed is stable. (i)
(BE =Bonds energy ) Formation of is exothermic thus is feasible. (ii) Formation of is endothermic, thus is not feasible.