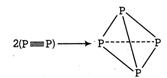
Q. Given the following bond dissociation enthalpies $ \left( \text{kJ mo}{{\text{l}}^{\text{-1}}} \right) $ . $ P\equiv P-490,P-P-209, $ $ N\equiv N-946, $ $ N-N-160 $ Compare the enthalpy changes for the process (i) $ 2{{P}_{2}}(g)\to {{P}_{4}}(g) $ (ii) $ 2{{N}_{2}}(g)\to {{N}_{4}}(g) $ Choose the correct option.
Bihar CECEBihar CECE 2015
Solution:
The process involves replacement of $ \text{2}\left( \text{X}\equiv \text{X} \right) $ units by 6 X?X bonds in the $ {{X}_{4}} $ tetrahedron. Energy is released when bonds are formed and thus species formed is stable. (i) $ 2{{P}^{2}}(g)\to {{P}_{4}}(g) $
$ \Delta {{H}_{t}}=-6\times BE(P-P)+2BE(P\equiv P) $ (BE =Bonds energy ) $ =-6\times 209+2\times 490=-2\text{74 kJ mo}{{\text{l}}^{\text{-}}} $ Formation of $ {{P}_{4}} $ is exothermic thus is feasible. (ii) $ 2{{N}_{2}}(g)\to {{N}_{4}}(g) $ $ \Delta {{H}_{t}}=-6BE(N-N)+2BE(N\equiv N) $ $ =-6\times 160+2\times 946=+9\text{32 kJ mo}{{\text{l}}^{\text{-1}}} $ Formation of $ {{N}_{4}} $ is endothermic, thus is not feasible.