Q.
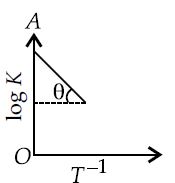
For a hypothetical reaction, a graph between and is straight line as follows, where and .
Assuming is independent of temperature, the equilibrium constants at and are respectively
Solution: