Q.
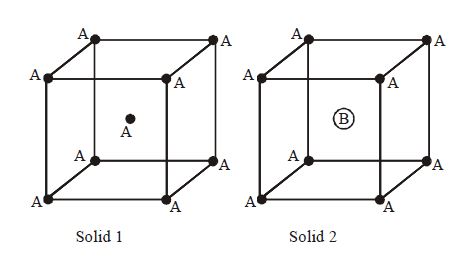
Consider the unit cells of the solids and with the position of atoms as shown below. The radius of atom is twice that of atom . The unit cell edge length is more in solid than in . What is the approximate packing efficiency in solid ?
Solution: