- Tardigrade
- Question
- Chemistry
- Consider a first-order decomposition process A 3 longrightarrow (3/2) A 2 <img class=img-fluid question-image alt=image src=https://cdn.tardigrade.in/img/question/chemistry/fffed4bf3e1bf41d87ad29630fa421f5-.png /> A plot of concentration of A 3 and A 2 versus time is shown below. At time t A percentage of reactant composed is
Q.
Consider a first-order decomposition process
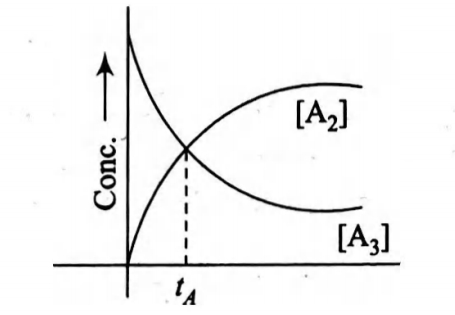
A plot of concentration of and versus time is shown below. At time percentage of reactant composed is
Solution: