Q.
Consider a first-order decomposition process
$A _{3} \longrightarrow \frac{3}{2} A _{2}$
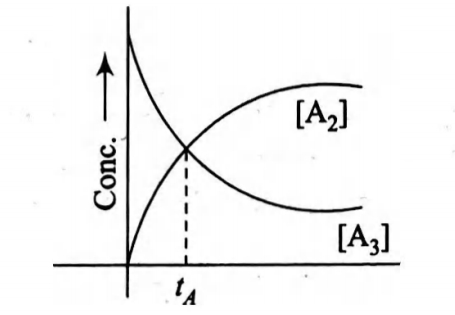
A plot of concentration of $A _{3}$ and $A _{2}$ versus time is shown below. At time $t_{ A }$ percentage of reactant composed is
Chemical Kinetics
Solution: