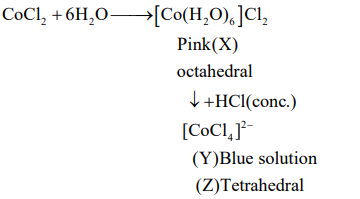
Q. Cobalt chloride when dissolved in water forms pink colored complex which has octahedral geometry. This solution on treating with cone forms deep blue complex, which has a geometry. and , respectively, are
Solution:
Correct answer is (c) Tetrahedral