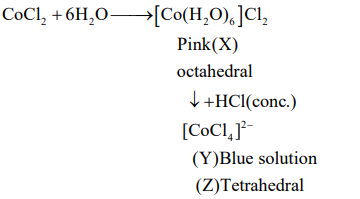
Q. Cobalt chloride when dissolved in water forms pink colored complex $X$ which has octahedral geometry. This solution on treating with cone $HCl$ forms deep blue complex, $\underline{Y}$ which has a $\underline{Z}$ geometry. $X, Y$ and $Z$, respectively, are
Solution:
Correct answer is (c) $X =\left[ Co \left( H _2 O \right)_6\right]^{2+}, Y =\left[ CoCl _4\right]^{2-}, Z =$ Tetrahedral