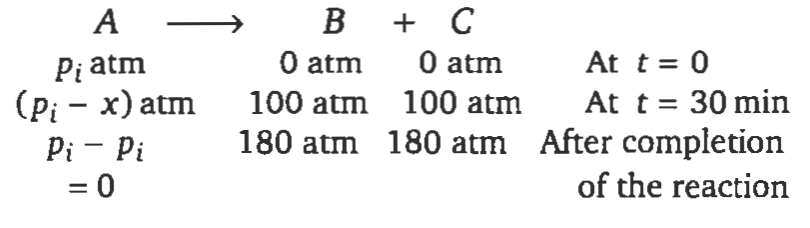- Tardigrade
- Question
- Chemistry
- At 300 K, a gaseous reaction: A arrow B + C was found to follow first order kinetics. Starting with pure A, the total pressure at the end of 20 minutes was 100 mm of Hg. The total pressure after the completion of the reaction is 180 mm of Hg. The partial pressure of A (in mm of Hg) is
Q.
At , a gaseous reaction:
was found to follow first order kinetics. Starting with pure , the total pressure at the end of minutes was . The total pressure after the completion of the reaction is . The partial pressure of is
Solution: