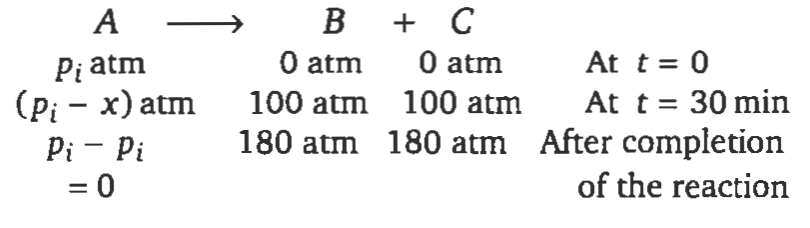Q.
At $300\, K$, a gaseous reaction:
$A \rightarrow B + C$
was found to follow first order kinetics. Starting with pure $A$, the total pressure at the end of $20$ minutes was $100\, mm \,of\, Hg$. The total pressure after the completion of the reaction is $180\, mm\, of\, Hg$. The partial pressure of $A (in\, mm \,of\, Hg)$ is
Solution: