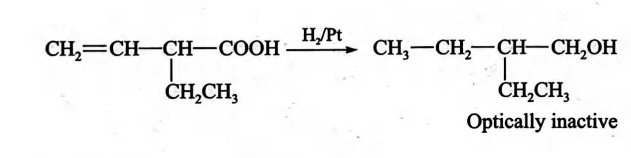- Tardigrade
- Question
- Chemistry
- An optically active carboxylic acid X has the molecular formula C 6 H 10 O 2 and can be shown by chemical and physical tests to contain the group - CH = CH 2 and atleast one - CH 3 group. When X is reduced with hydrogen over platinum, the product formed is optically inactive. Which of the following best represents the structure of X ?
Q. An optically active carboxylic acid has the molecular formula and can be shown by chemical and physical tests to contain the group and atleast one group. When is reduced with hydrogen over platinum, the product formed is optically inactive. Which of the following best represents the structure of ?
Solution:
Correct answer is (b)