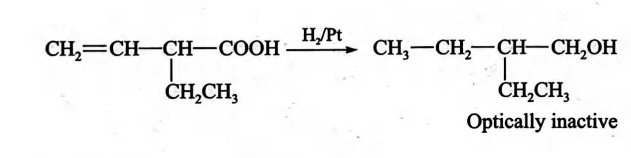Q. An optically active carboxylic acid $X$ has the molecular formula $C _{6} H _{10} O _{2}$ and can be shown by chemical and physical tests to contain the group $- CH = CH _{2}$ and atleast one $- CH _{3}$ group. When $X$ is reduced with hydrogen over platinum, the product formed is optically inactive. Which of the following best represents the structure of $X$ ?
Organic Chemistry – Some Basic Principles and Techniques
Solution:
Correct answer is (b) $CH _{2}= CH - CH \left( CH _{2} CH _{3}\right)- COOH$