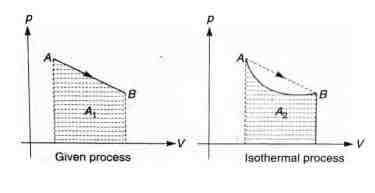
Q. An ideal gas is taken from the state (pressure , volume ) to the state (pressure , volume ) along a straight line path in the diagram. Select the correct statements from the following
Solution:
(a) Work done = Area under graph
(b) In the given process equation will be of a straight line with negative slope and positive intercept i.e.,
(Here and are positive constants)
...(i)
This is an equation of parabola in and
(d)
Now,
ie, has some maximum value.
Now, and
We conclude that temperatures are same at and and in between temperature has a maximum value.
Therefore, in going from to will first increase to a maximum value and then decrease.