Q. An ideal gas is taken from the state $A$ (pressure $p$, volume $V$) to the state $B$ (pressure $p /2$, volume $2 V$ ) along a straight line path in the $p - V$ diagram. Select the correct statements from the following
IIT JEEIIT JEE 1993Thermodynamics
Solution:
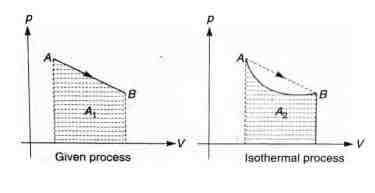
(a) Work done = Area under $p- V$ graph
$A_1 > A_2$
$\therefore W_{\text{given process} > W_{\text{isotermal process} }}$
(b) In the given process $p_V$ equation will be of a straight line with negative slope and positive intercept i.e.,
$p=-\alpha V +\beta$ (Here $\alpha$ and $\beta$ are positive constants)
$\Rightarrow p_V =- \alpha V^2 +\beta V$
$\Rightarrow nRT =-\alpha V^2 +\beta V$
$\Rightarrow T=\frac{1}{nR}(-\alpha ^2 +\beta V)$...(i)
This is an equation of parabola in $T$ and $V$
(d) $\frac{dT}{dV}=0-\beta -2\alpha V$
$\Rightarrow V=\frac{B}{2\alpha}$
Now, $\frac{d^2 T}{dV^2 =-2\alpha =-ve}$
ie, $T$ has some maximum value.
Now, $T \propto pV$ and $(pA)_A =(pV)_B$
$\Rightarrow T_A =T_B$
We conclude that temperatures are same at $A$ and $B$ and in between temperature has a maximum value.
Therefore, in going from $A$ to $B,\, T$ will first increase to a maximum value and then decrease.