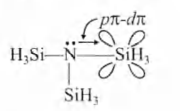- Tardigrade
- Question
- Chemistry
- Account for the following. Write the answers in four or five sentences only. (i) The experimentally determined N-F bond lengths in NF3 is greater than the sum of the single bond covalent radii of N and F. (ii) Mg3N2 when reacted with water gives of NH3 but HCl is not obtained from MgCl2 on reaction with water at room temperature. (iii) (SiH3)3 N is a weaker base than (CH3 )3 N.
Q.
Account for the following. Write the answers in four or five sentences only.
(i) The experimentally determined bond lengths in is greater than the sum of the single bond covalent radii of and .
(ii) when reacted with water gives of but is not obtained from on reaction with water at room temperature.
(iii) is a weaker base than
Solution: